XERMELO is recommended as a drug of choice in uncontrolled CSD patients on an SSA1
NANETS Guidelines recommend telotristat ethyl (XERMELO) as the appropriate drug of choice, in combination with SSA, for patients with stable radiographic disease and refractory CS characterized by suboptimal control of diarrhea. Under these circumstances, XERMELO was considered a more appropriate choice than an increase in SSA dose, use of short-acting octreotide, or use of nonspecific antidiarrheal or antitumor therapy.1
In situations where there is uncontrolled diarrhea, toward the end of the month/4 weeks/dosing cycle, other recommendations may apply.
XERMELO efficacy and safety profile
In the pivotal trial TELESTAR, XERMELO + SSA delivered superior reductions in BM frequency vs SSA alone over 12 weeks (primary endpoint; P<0.001).2

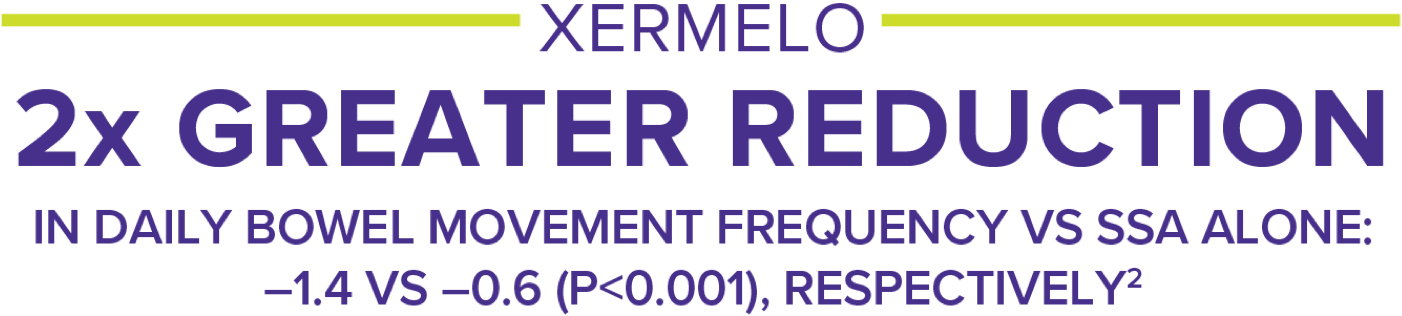
- Allow XERMELO 12 weeks to work, although some patients will see a response within 3 weeks2
89%
OF PATIENTS SHOWED IMPROVEMENT IN BOWEL MOVEMENT (BM) FREQUENCY WITH XERMELO + SSA2

69%
WITH SSA ALONE AT 12 WEEKS2
In TELESTAR, the average use of rescue short-acting SSA injections over the study period was 0.3 vs 0.7 for XERMELO + SSA vs SSA alone, respectively.2
In a post hoc analysis, the percentage of patients with significant* improvement with XERMELO + SSA grew to 75% over 12 weeks vs 55% with SSA alone.3


*Defined as a sustained response, starting with the first day of 2 consecutive weeks with a mean BM frequency improvement of ≥30% from baseline.
Real-world evidence demonstrates consistent benefit of XERMELO for improving CSD4
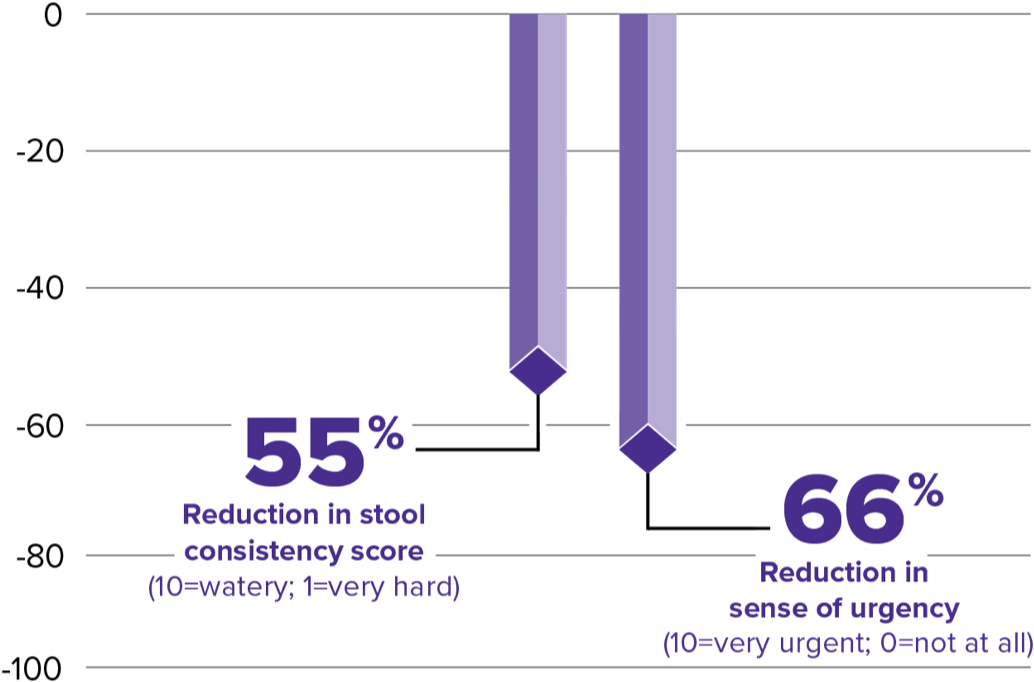
After 3 months of XERMELO treatment, patients reported significant reductions in daily bowel movement (BM) frequency, sense of urgency, and improved stool consistency
64%
MEAN DAILY BM REDUCTION
(from 6.3 at baseline to 2.3)
REDUCED BY NEARLY 4 BMs PER DAY
OF THE 1603 PATIENTS WHO INITIATED XERMELO DURING THE STUDY PERIOD, 684 PATIENTS ENROLLED IN THE NURSING SUPPORT PROGRAM AND STAYED ON TREATMENT FOR 3 MONTHS.
Patients reported a -3.66 mean reduction in stool consistency score and -5.42 mean reduction in urgency.

In the pivotal trial, TELESTAR, patients taking XERMELO 250 mg + SSA (n=45) had a numerical improvement in stool consistency (mean treatment difference -0.09; P=0.57) and urgency (mean treatment difference -0.02; P=0.35) versus patients taking SSA alone, but this did not reach statistical significance.5
XERMELO + SSA was safe and well tolerated in most patients in the pivotal trial population2
In this trial, the incidence of adverse events was similar among patients treated with XERMELO + SSA and those treated with SSA alone.2
COMMON ADVERSE REACTIONS* BY TREATMENT GROUP IN A DOUBLE-BLIND PLACEBO-CONTROLLED TRIAL OF PATIENTS WITH CSD AT WEEK 122


XERMELO may cause constipation, which can be serious. Serious complications of constipation have been reported during clinical trials and post marketing with individual reports of intestinal perforation, obstruction, and fecaloma.
*Incidence of ≥5% in the XERMELO treatment group and at an incidence greater than SSA alone.2
LOWER DISCONTINUATION RATES WERE SEEN WITH XERMELO + SSA COMBINATION THERAPY5
Treatment-emergent discontinuation rates were low across all treatment groups5
- 7% with XERMELO + SSA
- 13% with SSA alone


Request a XERMELO representative
A representative can give you more information about XERMELO and provide resources to support your patients.
Request a repSupport for your patients
Get support and resources to help your patients on their treatment journey.
Download practice resources
Download patient brochures, enrollment forms, sample prior authorization letters, checklists, and more.
References:
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46(6):707-714.
- XERMELO® (telotristat ethyl) Prescribing Information. TerSera Therapeutics LLC.
- Dillon JS, Kulke MH, Hörsch D, et al. Time to sustained improvement in bowel movement frequency with telotristat ethyl: analyses of phase III studies in carcinoid syndrome. J Gastrointest Cancer. 2021;52(1):212-221.
- Kulke MH, Kennecke HF, Murali K, Joish VN. Changes in carcinoid syndrome symptoms among patients receiving telotristat ethyl in US clinical practice: findings from the TELEPRO-II Real-World Study. Cancer Manag Res. 2021;13:7439-7446.
- Kulke MH, Hörsch D, Caplin ME, et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol. 2017;35(1):14-23.